25+ Calculating Excess Reactant
For example if the balanced equation. 12 mol ClO2 x 1 mol Br2 6 mol ClO2 2 mol Br2.

How Do You Determine How Much Of The Excess Reactant Is Left Over Also How Do You Determine How Much More Of The Limiting Reagent Would You Need To Use Up The
Our final step is to determine the theoretical yield of ce AlCl_3 AlCl3 in the reaction.

. The math would look as follows. How to Find Excess Reactant Assume a reaction that commences with 10 moles of hydrogen gas H2 H 2 and 7 moles of oxygen gas O2 O 2. 2 Rb s MgCl2 s Mg.
The coefficients of a. 2Na sCl 2 g2NaCl s Then we will calculate the molecular mass of each reactant. How to find limiting reagent and excess reactant for the limiting reactant equation given below.
To calculate the excess reactant firstly we will balance the chemical reaction. Calculate the mass of excess reactant used up. The excess reactant may be found using the balanced chemical equation for a reaction which gives the mole ratio between reactants.
Remember that the theoretical yield is the amount of product. Answer 1 of 4. Which reactant is in excess when 025 g of magnesium is added to 100 cm3 of 01 mol l-1 hydrochloric acid HCl.
Divide the amount of moles you have of each reactant by the coefficient of that substance. 100g 86936 molg 3. The reactant that produces a larger amount of product is the excess reagent.
5 mol BrF3 x 1 mol Br2 2 mol BrF3 25 mol Br2. To find the amount of remaining excess reactant subtract the mass of excess reagent. Use the molar ratio from the equation to convert moles of O2 from Step 3 to moles of NH3 and then convert moles of NH3.
It shows you how to perform stoichiometric calculations an. Start with a balanced equation. N2 H2 NH3 Solution.
Limiting Reactant and Mass of Excess Reactant 500 g Rb are combined with 344 g MgCl 2 according to the chemical reaction. Calculate the theoretical yield. Reactant grams1 x 1 mol reactant reactant grams x 2 mol product4 mol reactant x product grams1 mol product.
As the given reaction is not balanced so its balanced form is as. Since BrF3 produces more moles of Br2 this is the excess reactant. Check if there is any information regarding the amount of.
For example lets assume we have 100g of both MnO2 and Al. So if you follow it you can see each. If already balanced then leave it if not then balance it appropriately.
This chemistry video tutorial shows you how to identify the limiting reagent and excess reactant. Mg 2HCl rightarrow MgCl_ 2.
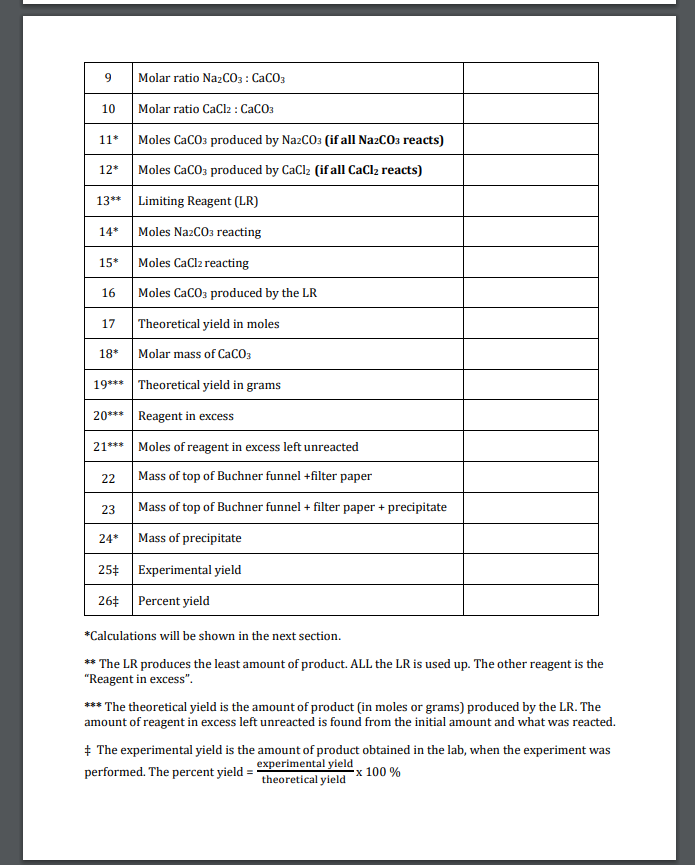
Solved Data For Experiment 11 Introduction To Precipitation Chegg Com
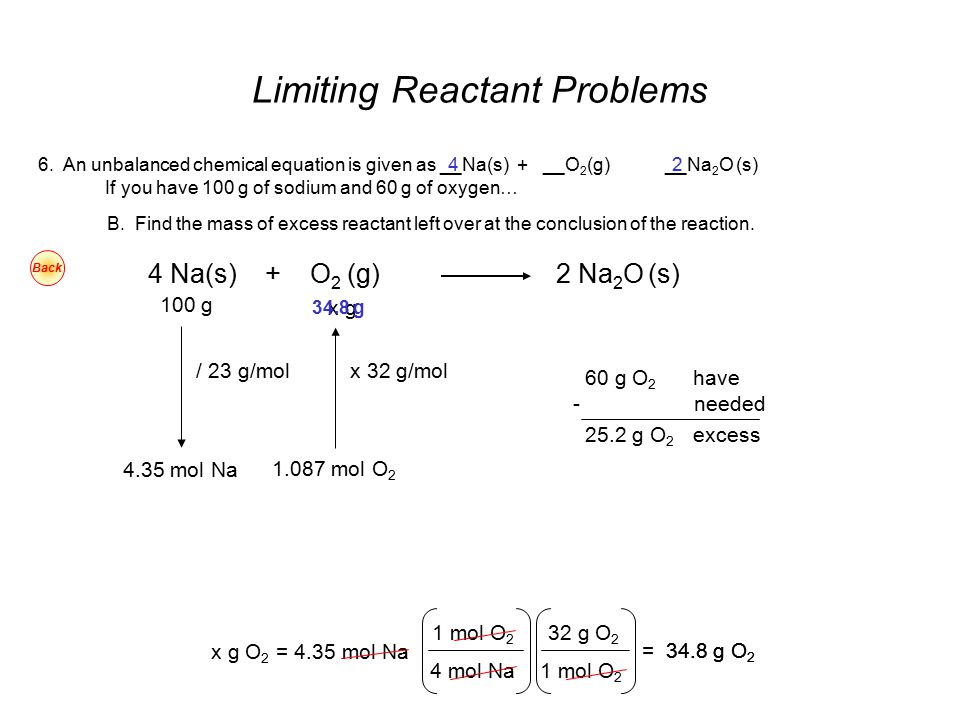
Excess Reactant Ppt Video Online Download

How To Calculate The Amount Of Reactant In Excess Sciencing

Calculating Mass Of Excess Reactant Leftovers In Limiting Reactant Problems Tutorial Youtube

Grade 11 Chemistry Quiz Solid Stoichiometry Limiting Reactant Excess Reactant Quiz With Answers 9 Teaching Resources

4 2 Limiting Excess Reagents Chemistry Libretexts

4 2 Limiting Excess Reagents Chemistry Libretexts
Limiting And Excess Reactants In Chemistry
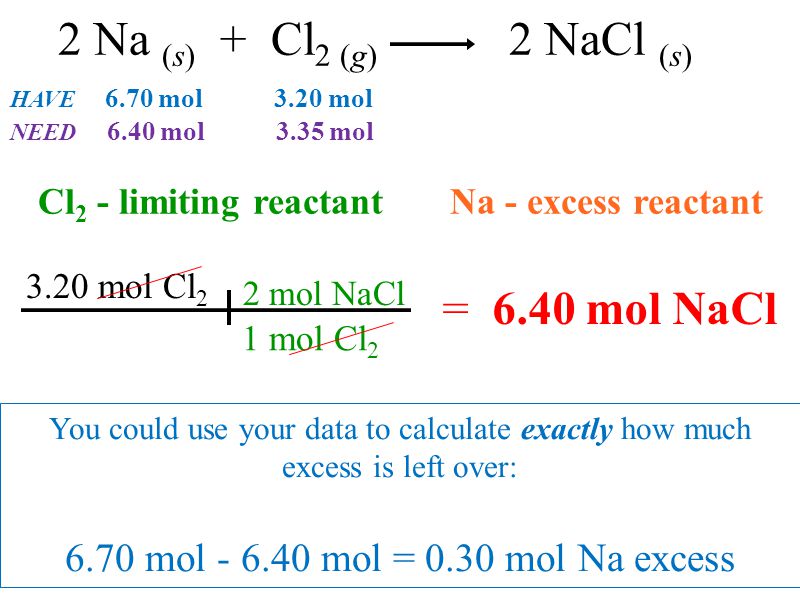
Limiting Reactant Ppt Download
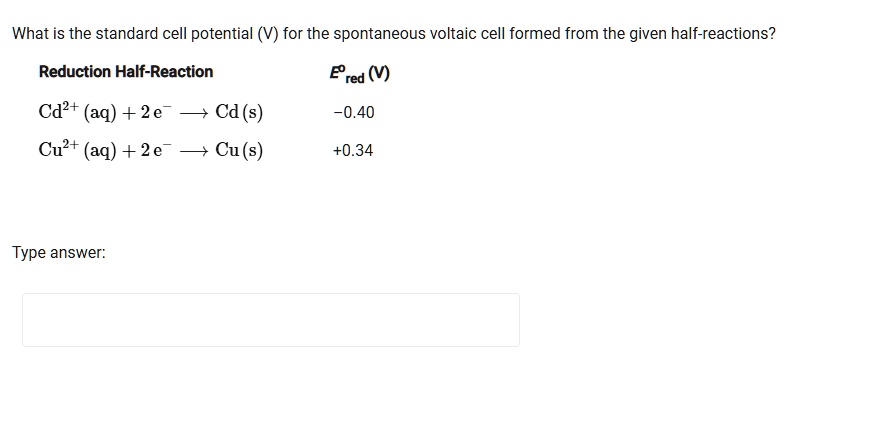
Browse Questions For Chemistry 101

Limiting And Excess Reactants In Chemistry

Chapter 6 Gases

Calculate Limiting Reagents Excess Reagents And Products In Chemical Reactions Dummies

Finding The Amount Of Excess Reactant Left Over Ppt Download

Limiting And Excess Reagents Vce Chemistry

Secondary Organic Aerosol Mass Yields From No3 Oxidation Of A Pinene And D Carene Effect Of Ro2 Radical Fate The Journal Of Physical Chemistry A

Determining The Mole Ratio In A Chemical Reaction Chemistry Ppt Download